Dr Larry M Games
Vice President
Procter and Gamble Pharmaceuticals
Health Care Research Center
8700 Mason-Montgomery Road
Mason, Ohio, 45040
USA
23 November 2006
Re: Actonel Studies/Sheffield Situation - Issue of Raw data
Dear Dr Games
I am writing following our previous correspondence. We have a number of matters to discuss. To maintain clarity I will write separately about each matter. The purpose of this letter is to revisit your earlier refusal to allow the raw data you provided in April 2006 to be made available for public/scientific scrutiny.
I again thank you for your eventual provision in April 2006 of raw data underlying the three intended P&G Publications (Eastell et al. 2003 J.Bone.Miner.Res. 18:1051-6 + the two abstracts/draft publications in my name). I also thank you for your commitment to openness, and for writing the Bill of Rights for researchers. All of this is good.
Unfortunately the Eastell 2003 publication (J.Bone.Miner.Res. 18:1051-6) has not yet been retracted.
As stated, the main purpose of this letter is to revisit your earlier refusal to allow the data provided in April to be scrutinized in an open manner. Your refusal to allow the data to be transmitted to a journal editor accompanying a properly corrected manuscript is not appropriate. A journal editor can request raw data from an author at any time, and such refusal (particularly under the circumstances of this case) would be inappropriate.
The raw data you sent me by E-mail in April is attached exactly as sent (PGData.zip - web downloadable data is encrypted in the online letter). The CD-Rom and paper versions of the data you sent are retained by my legal representative.
Obviously the refusal to supply the raw data to authors in the first place has caused some international consternation. There is also the entirely separate matter concerning the potential mis-analysis of data by P&G in the ghost-analyzed/ghostwritten material. I believe the data was mis-analyzed - and quite obviously so. Two statisticians agree with me.
Certainly data must be transmissible to journal editors. This whole matter could be settled most easily by making the full data as attached available for open scrutiny. Secrecy has no further place in this, and I am asking you to allow this. Any other approach is I am afraid not going to satisfy anyone.
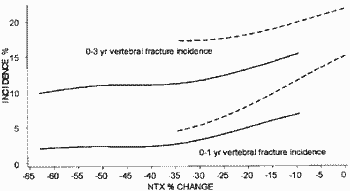
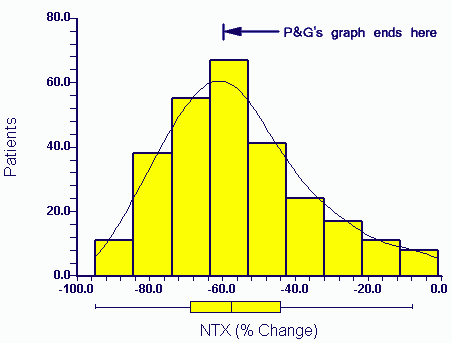
You also make the unusual comment in your last letter that the Eastell 2003 article is somehow separate from the other two intended publications and abstracts in terms of data. Obviously you must know that this is not true. You yourself provided me with the data underlying the Eastell 2003 paper (PGData.zip - encrypted). One of the two manuscripts in my name includes the entire Eastell 2003 data (for NTX and fractures) as a subset since it was based on a combined analysis of the HIP and VERT studies. The related abstract in my name involving the Eastell data (as a subset) is referenced below (1). I am sure you have the draft publication of 2003 relating to this abstract.
You will also no doubt be aware of (and have seen) the statistical reports which attempt to reconstruct Figure 1 and related statistics in the manuscript by Richard Eastell (2003) from the data. One of these reports is here (Professor Martin Bland). Obviously there were several other problems with the analysis in all manuscripts but for the moment I will keep things simple.
There is a high road Dr Games, and I hope that we can work together to take it.
Kind wishes
Dr Aubrey Blumsohn
MBBCh, BSc (hons), MSc, PhD, MRCPath
Reference:
1. A. Blumsohn, IP Barton, A Chines, R Eastell Relative Contributions Of The Early Changes In Bone Resorption And Later Changes In Hip Bone Mineral Density To The Reduction In Vertebral Fracture Risk With Risedronate. J Bone Miner Res 2003;18(S2):S157 Abst#SA337
Earlier|Later|Main Page

 Editors wanting hints as to how to react when approached with concerns about research misconduct involving a major pharmaceutical sponsor and fellow friendly scientists, see the
Editors wanting hints as to how to react when approached with concerns about research misconduct involving a major pharmaceutical sponsor and fellow friendly scientists, see the 
 Fellow medical bloggers are writing about the way in which the pharmaceutical industry is preventing proper understanding of the science upon which our patients rely (
Fellow medical bloggers are writing about the way in which the pharmaceutical industry is preventing proper understanding of the science upon which our patients rely ( Odette writes: Having had some contact with the world of scientific research, I had just assumed that fraud was standard practice and that no scientist would put good money at risk by publicly admitting that his basic assumptions were wrong when he started planning an experiment. You can't just lose money in blind alleys and you can't be at the mercy of your data. Time and again, I have seen researchers replacing missing data cells with the group average, removing outliers until they get the variance they want, doing linear transformations and even using mind-boggling statistical techniques of their own invention to get a p-value worth publishing.
Odette writes: Having had some contact with the world of scientific research, I had just assumed that fraud was standard practice and that no scientist would put good money at risk by publicly admitting that his basic assumptions were wrong when he started planning an experiment. You can't just lose money in blind alleys and you can't be at the mercy of your data. Time and again, I have seen researchers replacing missing data cells with the group average, removing outliers until they get the variance they want, doing linear transformations and even using mind-boggling statistical techniques of their own invention to get a p-value worth publishing.









 Pending further description of methodology in
Pending further description of methodology in