Much has been written about the problem of "astroturf" patient advocacy groups (
1,
2,
3,
4,
5,
6). These are patient "support" groups with a facade of grassroots advocacy - but with real interests that lie elsewhere (
7).
It isn't hard to discern fake advocacy. The cracks show when concerns are raised about scientific dishonesty, hiding of evidence, and regulatory malfunction. Patients rely on honest independent science. Legitimate advocacy groups (such as
MIND ) show concern and
outrage when there is evidence of threat to the integrity of science upon which their patient "clients" depend. Mute behavior of an "advocacy" group provides evidence of illegitimacy.
Less widely discussed is the inclination of some "advocacy" groups to intimidate individual scientists, clinicians or patients who wish to discuss concerns about scientific integrity.

For a good example of this, I return to my ongoing investigation of the
Gillberg Affair. Dr Leif Elinder (left) is one of the two individuals who raised serious concerns about the veracity of the study findings and patient consent in studies reported by the Gillberg team in Sweden. Elinder is an Uppsala pediatrician specialising in the care of children with complex educational needs. He also expressed concerns about poor science and industry influence underlying the diagnosis of ADHD (as have
many others). During these events the Gillberg team destroyed all their raw data, preventing any exploration of alleged research misconduct (for details see
here and
here). This followed a court order to allow proper and confidential scrutiny of the records by investigators. The Gillberg team provided a
laughable rationale for their destruction of those data and their prevention of scrutiny. A key aspect of the science involving the diagnosis of ADHD was thereby placed into considerable doubt. Any legitimate advocacy group would have been outraged (likewise all honest psychiatrists).
The Attention Society (
Riksförbundets Attention) is the Swedish society supposedly advocating for children with ADHD (UK equivalent of
ADDISS, or the US
CHADD).
What did these advocacy groups do?
Well the facts stand for themselves:1) Riksförbundets Attention did not criticize the actions of the Gillberg team. Neither to my knowledge did ADDISS or CHADD.
2) Riksförbundets Attention instead started a financial collection on their
website in support of legal expenses for Christopher Gillberg [Insamling till stöd för Christopher Gillberg]
3) Riksförbundets Attention on their
website resorted to the dismal technique of implying that Elinder is a Scientologist, which (even if relevant) he is not. They state: "När det gäller medicinering som är det stora svarta skynket för dessa personer, vars åsikter ligger nära scientologerna" [which is the real bad thing for these people, whose views are close to the Scientology movement].
4) They then placed a formal complaint with the National Board for Education and the body that licenses doctors in Sweden about Elinder (not Gillberg).
Swedish Radio 17 Aug 2006
reported that:
Ann-Kristin Sandberg, Chairman for the Riksförbundet Attention had approached both The National Board of Social Welfare (Socialstyrelsen, the body that registers doctors in Sweden) to "investigate whether the Uppsala physician Leif Elinder, known for his controversial views on ADHD should be allowed to keep his doctors certificate". "The organisation has the view that the National Board of Social welfare (Socialstyrelsen) should investigate Elinders suitability as a physician" "This spring he wrote an article for the newspaper in Uppsala where he called ADHD concept a horoscope. This was the final straw and it was taken as an insult towards our members who are suffering from the symptoms."
5) Similarly the Swedish newspapers (
Publicerad: 2006-06-08) reported that:
"The view of the Attention society who strongly attacks Leif Elinder who calls ADHD a horosope concept. For this reason the Attention society, an interest organisation for people with neuropsychiatric disabilities such as ADHD have sent a petition to Skolverket to attempt to consider whether Elinder can pursue his work in his field"
6) Riksförbundets Attention wrote to Socialstyrelsen in an attempt to get Elinder struck off. That letter is here (click on images for larger versions):

Socialstyrelsen replied quite appropriately telling Riksförbundets Attention to get lost:

7) Gillberg as well as his wife (Carina) wrote intimidating letters to about a dozen member's of Elinder's family, but Riksförbundets Attention didn't comment on the appropriateness of this either.
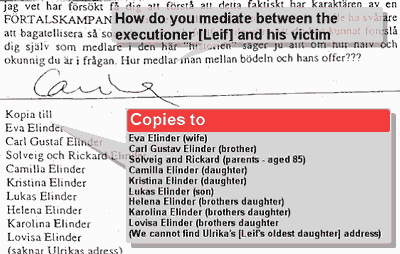
8) In the meantime the
website of Riksförbundets Attention states that they get extensive funding from a variety of pharmaceutical companies. Dr Björn Kadesjö is one of three members of their
medical advisory board. This same Kadesjö has co-signed commercial pharmaceutical confidentiality agreements with Gillberg (
link). Kadesjö has published 14 papers with Gillberg about ADHD (
link) constituting Kadesjö's entire research output.
Woe be to all of us.
Earlier|
Later|
Main Page
 I found this book interesting.
I found this book interesting.  I
I  I introduce two new weekly features on this blog.
I introduce two new weekly features on this blog. 
 For a good example of this, I return to my ongoing investigation of the
For a good example of this, I return to my ongoing investigation of the 


 I have written
I have written  Think of your favorite teddy bear. Now imagine it’s been torn apart, disemboweled, and turned inside-out. That’s what artist Kent Rogowski’s has done in his excellent Bear series. He mangles our memories of the world.
Think of your favorite teddy bear. Now imagine it’s been torn apart, disemboweled, and turned inside-out. That’s what artist Kent Rogowski’s has done in his excellent Bear series. He mangles our memories of the world.
 Professor Christopher Gillberg was Professor of child psychiatry at Gothenburg University, in Sweden. He is visiting Professor of psychiatry at the
Professor Christopher Gillberg was Professor of child psychiatry at Gothenburg University, in Sweden. He is visiting Professor of psychiatry at the 