8 years ago today: A story of ducks and a single death in a Vioxx clinical trial
On 21 October 1999 a 73-year-old woman died. That death and our response to it tells volumes about the health of our profession.In medicine we sell our wares under the banner of science. The technique of science (the "scientific method") consists of a number of steps. For a double blind randomized clinical drug trial those steps might be:
- State a meaningful hypothesis
- Design a study and statistical methods to test it
- Do the study in a double blind randomized way
- Unblind the study AFTER deciding what happened to each patient and locking the database
- Study authors analyze the results
- Share the results with everyone
So that takes us to the Merck Advantage trial of Vioxx, and a single 73-year-old female participant in that trial who died on this day - Oct. 21, 1999. She died a few minutes after calling her son to tell him she felt short of breath.
The Advantage trial was a very short (12-week) clinical trial in 5,500 patients. If you want to avoid showing up long term side effects, you keep your clinical trials short and scientifically irrelevant (that's about the meaningful hypothesis part).
Now eight people taking Vioxx suffered heart attacks or sudden cardiac death in the Advantage trial, compared with just one taking naproxen, according to data released by the F.D.A. in 2005. The difference was statistically significant, but Merck never showed the data that way.
In the published study, Dr. Lisse (University of Arizona) reported that five patients taking Vioxx had suffered heart attacks during the trial, compared with one taking naproxen, a difference that did not reach statistical significance. But the paper never mentioned the other cardiac deaths of patients taking Vioxx, including the 73-year-old woman.
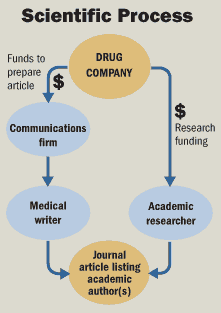
Dr. Lisse said that while he was listed as the paper's first author, Merck actually wrote the report (that's the authoring bit). "Merck designed the trial, paid for the trial, ran the trial," Dr. Lisse said. "Merck came to me after the study was completed and said, 'We want your help to work on the paper.' The initial paper was written at Merck, and then it was sent to me for editing."
Now, the other thing in usual science is not to allow company technocrats with a vested interest to decide themselves what happened to individual 73 year old women after the study is unblinded.
Neither patients nor their doctors were aware whether patients were receiving Vioxx or naproxen. Results from the trials were also supposed to be blinded when they were examined, so that Merck researchers could not bias the results. After examining the case, Dr. Eliav Barr, a Merck employee judged that the woman had probably died of a heart attack."
"Common things being common, the clinical scenario is likely to be MI (heart attack)" Dr. Barr wrote in an E-mail message in November 2000 to Dr. Reicin, the Merck clinical research executive.
Dr. Reicin quickly shot back: "I think this should be called an unknown cause of death." A few hours later, she wrote, "I would prefer unknown cause of death so we don't raise concerns."
 "A fatal event not being treated as objectively as possible surprises me," said Dr. Myerburg, a prominent cardiologist. "It looks like they're playing around with this death."
"A fatal event not being treated as objectively as possible surprises me," said Dr. Myerburg, a prominent cardiologist. "It looks like they're playing around with this death."In November 2001, an F.D.A. reviewer who had examined all the company's data concluded in a report to other agency officials that Merck had misclassified the death. But the F.D.A. never told anyone either.
Dr. Lisse, the study "author" said he had never heard of the case of the woman who died, until told of it by a reporter.
So can we really believe anything at all, and how to distinguish this from the worst type of quackery? As the old saying goes, ...If it looks like a duck, and it quacks like a duck...it's a bloody duck.
What is even more amazing is that the leadership of medicine says nothing -- nothing at all. Medical students learn none of this in any of their pseudo-ethics training.
Sources:
- Correspondence in the Annals of Internal Medicine on the problem: Deeply disturbing evidence of medicine run amok
- New York Times, April 24, 2005: "Evidence in Vioxx Suits Shows Intervention by Merck Officials"
3 comments:
"...Check out this informative article I found where you can learn more about preventing heart attacks."
Hint #1: try to avoid drugs that exacerbate existing conditions.
Matt
"Mercky" data!
A whole set of new words
Mercky (adj) - as in Mercky data
Adverb - To Merck data
Noun - What a Merck
Expletive - Oh my Merck
Verb - he Mercked
Please keep writing about "Merck",
There is not enough said about this company, They are not taking responsibilty for there actions, with VIOXX, what kind of responsiblity with they take with "Gardsil?, and that means our children are at risk!!!!
Nancy
Post a Comment