 This news-snippet rings a few alarm bells. I have posted about some apparently dodgy dealings in a clinical trial involving Boston-based NMT Medical, the MIST I trial, and UK clinical investigators.
This news-snippet rings a few alarm bells. I have posted about some apparently dodgy dealings in a clinical trial involving Boston-based NMT Medical, the MIST I trial, and UK clinical investigators.The previous saga involved a medical device, and the conduct and conclusions of the Migraine Intervention with STARflex Technology (MIST) I trial. MIST I had two co-principal investigators:
- Dr Peter Wilmshurst, a respected cardiologist and
- So-called headache specialist Dr Andrew Dowson. Although Dowson described himself as a neurologist, he is not. More importantly, Dowson has restrictions on his medical practice following previous research misconduct.
Several ethical, procedural and scientific issues arose in MIST I which went beyond the issue of migraine to other aspects of the device. One can only hope that NMT are not going be so foolish as to attempt to threaten Dr Wilmshurst for quite properly discussing scientific procedures involving patients. The of MIST I is ongoing, and it seems that NMT may have resorted to legal threats - always a credible scientific tactic. This case is worth keeping on the radar.
Now we read that NMT is shutting down the MIST II trial. This may be appropriate as MIST I showed no efficacy for migraine. However questions have to be asked when trials are shut down. If the continuation of MIST II was in any way dependent on the outcome of MIST I, then the ethics of starting MIST II have to be questioned. If the termination was truly related to difficulty with recruitment then this raises other issues.
NMT Medical closes migraine trial in U.S. Mass High Tech, 23 Jan 2008
NMT Medical Inc. reports plans to shut down its migraine technology trial, MIST II, to instead focus on using its technology to prevent the onset of stroke.Earlier|Later|Main Page
According to officials at Boston-based NMT, the move should save the company approximately $14 million over the next two to three years, as it redistributes funds originally budgeted for MIST II (Migraine Intervention with STARFlex Technology). NMT has stopped enrolling patients in MIST II, which was being conducted at 20 centers in the United States.
NMT president and CEO John E. Ahern said that strict enrollment requirements from the U.S. Food and Drug Administration has made MIST II an expensive endeavor "with little likelihood of being completed in a reasonable time frame."
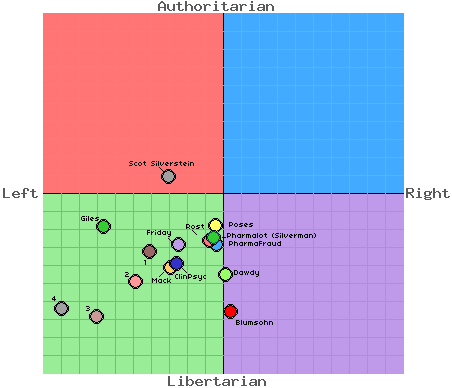




 Last year the US Congress required drug companies to post announcements of clinical studies in a public database.
Last year the US Congress required drug companies to post announcements of clinical studies in a public database.

 The famous biochemist and science fiction writer
The famous biochemist and science fiction writer 
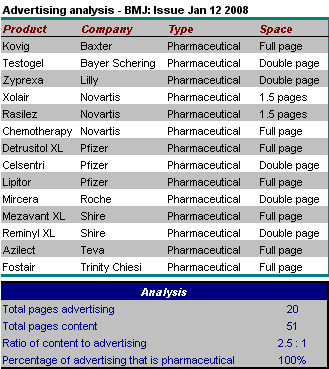
 A report in the British Medical Journal yesterday is fascinating:
A report in the British Medical Journal yesterday is fascinating: The huge ethical and scientific problems with the drug Ezetimibe have been discussed widely in the lay press (especially in the New York Times and some excellent reporting in Forbes). The problems have been discussed by patients, on the internet and in the blogsphere. However as of today, a search of our key medical journals reveals no hint of any ethical discussion. No discussion of the principles of good science. Not even a worthy news report. Nothing at all.
The huge ethical and scientific problems with the drug Ezetimibe have been discussed widely in the lay press (especially in the New York Times and some excellent reporting in Forbes). The problems have been discussed by patients, on the internet and in the blogsphere. However as of today, a search of our key medical journals reveals no hint of any ethical discussion. No discussion of the principles of good science. Not even a worthy news report. Nothing at all.  Several bloggers have been following the saga of Ezetimibe (Zetia, Vytorin), the hidden data, and the threats to science which have resulted from the way in which Merck and Schering-Plough have thrown the usual safeguards of science into the gutter.
Several bloggers have been following the saga of Ezetimibe (Zetia, Vytorin), the hidden data, and the threats to science which have resulted from the way in which Merck and Schering-Plough have thrown the usual safeguards of science into the gutter.
 Although not about medical scientific misconduct, this is directly related.
Although not about medical scientific misconduct, this is directly related. A new year, and I thought I would kick off with an irritating year-end piece from Wired magazine. On 27 December Wired ran an item reporting the supposed "10 most important scientific breakthroughs of 2007" (
A new year, and I thought I would kick off with an irritating year-end piece from Wired magazine. On 27 December Wired ran an item reporting the supposed "10 most important scientific breakthroughs of 2007" (