This tutorial does not address whether they "did it" but evaluates one proposed mechanism for "functional unblinding".
One mechanism is to examine the primary outcome variable for bimodality (two camel-like humps indicating separation of treatment arms). Is this possible? Some bloggers and imaging experts suggest that this might have happened in ENHANCE (See Brandweek, Pharmagossip).
Jack Friday explains the potential mechanism thus:
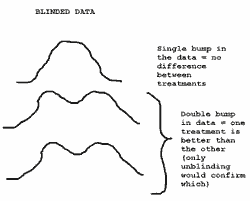
is this possible?
The short answer is no, at least for the primary response variable in a usual clinical trial. It all depends on study power. Usually, trials are sized so as to provide reasonable power (say 70% to 90%). The greater the power, the greater the chance of getting obvious bimodality in unblinded data. However, even at 95% power, bimodality in combined unblinded responses would never be a reliable indicator of a treatment response (or lack of response). It is possible to show this using complicated equations, but a simple graphical example with simulated data (based on the ENHANCE study) is easier.
Enhance had a prior declaration of power as follows:
![[Power statement]](http://www.thejabberwock.org/blog/2/pow1.png)
As discussed in Tutorial 04, this power calculation was slightly wrong. However leave that aside for now.
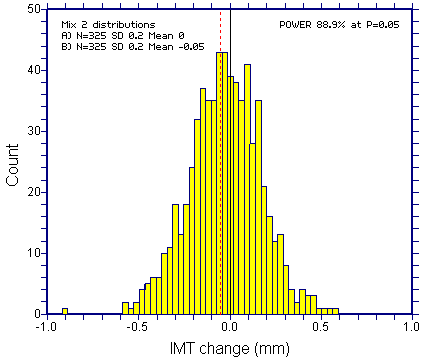
Assume that the study showed the desired effect of Vytorin. This would mean a "left" shift of 0.05mm (or one quarter of a standard deviation) in the Vytorin arm (with both arms having SD=0.2mm). The plot below shows the distribution of a mix of those two distributions, each at N=325. For convenience, the "placebo" distribution is centered at zero. The vertical dashed line shows the mean of the Vytorin distribution (for an outcome Schering Plough would have deemed as "positive"). This small change that would have been regarded as "positive" is interesting in its own right. Clearly, there is no evidence of bimodality on visual inspection. There are tests for bimodality, but these would not help much more than simple "eyeballing".
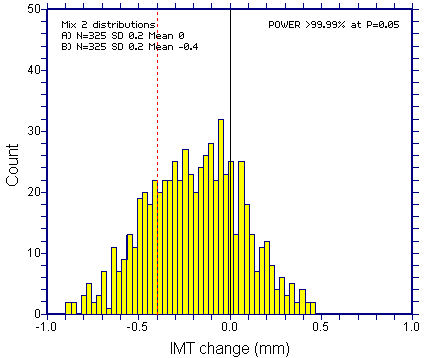
Even if the desired (or expected) treatment effect was eight times bigger at 0.4mm (2 x SD), it would be hard to be sure about bimodality (there would be broadening of the distribution, but this would provide dubious evidence). Such a study would have practically 100% Power (highly unlikely in any study powered on the primary outcome).
Bimodality would however become evident if the SD was halved to 0.1mm in each arm together with the eight fold increase in the treatment effect.
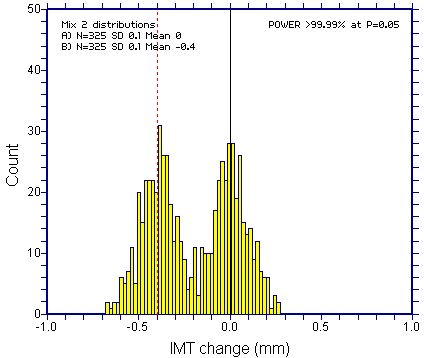
Bottom line: Bimodaility of response (for the primary outcome variable) is not going to be a reliable mechanism to "functionally unblind" a trial.
There are far better methods.
See here for Collated Micro-Statistics Tutorials
Earlier|Later|Main Page
1 comment:
Looking forward to the next tutorial.
Please see this:
http://bmartinmd.com/2008/01/enhance-study-basically-the-ul.html
Cheers
Jack
Post a Comment